Career Opportunities
At Eschmann, we believe that every individual can make a big difference.
We're passionate in seeking ambitious, talented and motivated individuals at every stage of their career, helping them to thrive and develop skills across a variety of roles.
Eschmann For You
Here are just some of the perks that we offer our employees...
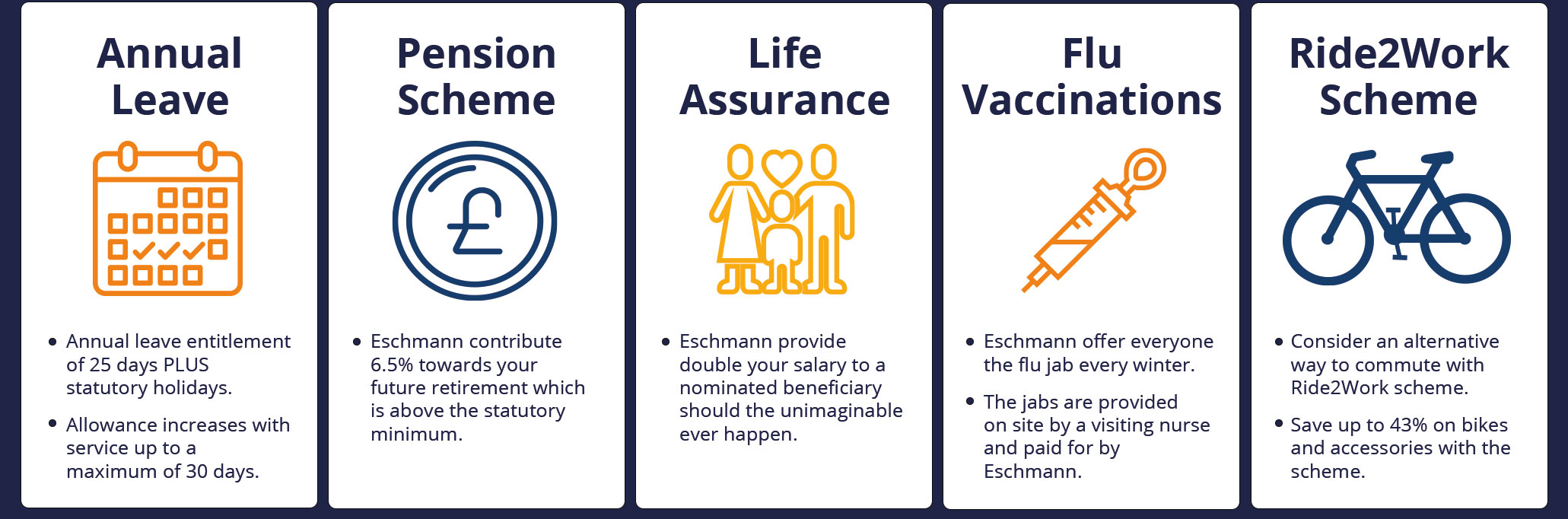

Job Openings
Please complete the form below to apply. If you can't see the vacancy you are looking for and would like to be considered for future roles, please send us your CV.
Job Title: Customer Support Adviser
Location: Lancing
Hours of work: 37.5 - 6 month temporary position with possibility of conversion to permanent role
Starting salary: £25,063 per annum
In this role you will be on the front line, receiving customer calls and emails relating to breakdowns and requests for services, you will be providing the first line of technical support to customers over the phone on the Eschmann range of products. You will be raising jobs, assigning them to relevant engineers, and liaising with Service Engineers as required.
The position, within the Service Support Team, falls within the overall Customer Support Department, which handles all orders, quotations, contracts and any issues regarding the services and breakdown of equipment.
About You
You will possess excellent customer service skills and demonstrate strong communication and interpersonal skills to both internal and external customers.
With a flexible approach you are confident in prioritising customer queries and providing appropriate responses.
For You
We're passionate in seeking ambitious, talented and motivated individuals at every stage of their career, and will provide them with the following:
- 25 days annual leave plus bank holidays, increasing to 30.
- Cycle2work scheme
- Money back on everyday health costs including optical, dental and other therapies
- Training and development support
- Retail and health club discounts
- Access to a 24-hour advice and Information line for you and your family covering legal, health and wellbeing advice
- Life Assurance
- Company sick pay scheme
As an SME, we work closely with one another, meaning you are likely to meet or work with everyone, including our senior team. Our green credentials form part of our core values, and we have a dedicated task force that meets and communicates regularly on our green agenda.
Our Story
Eschmann Technologies is a British design and manufacturing business providing medical, dental and animal health products and services to clinical professionals and organisations around the world, and we are proud of our history and heritage.
First established in 1830 and starting out in surgical instruments, we went on to use our experience to manufacture operating theatre tables, electrosurgery, surgical suction units and autoclaves. Today, we are proud to manufacture autoclaves and surgical suction devices which are used across a wide range of sectors including: healthcare, dental health, animal health and workplace solutions.
We have long recognised that it is the people who make us who we are and help us to make the difference. We operate with a team of over 40 trained engineers across the UK, guaranteeing you can always rely on Eschmann to provide high quality products as well as an unrivalled level of service.
Location and transport
Based in Eschmann House, Lancing, West Sussex on the sunny South coast, we have on-site parking for those using 4 wheels, Lancing Station is a 20-minute walk for those requiring train services, the nearest bus stop is a 5-minute walk, and for those wishing to use 2 wheels, bike storage and on-site shower facilities are available.
You can view our Recruitment Privacy Statement here.













